
What is Accutane™?
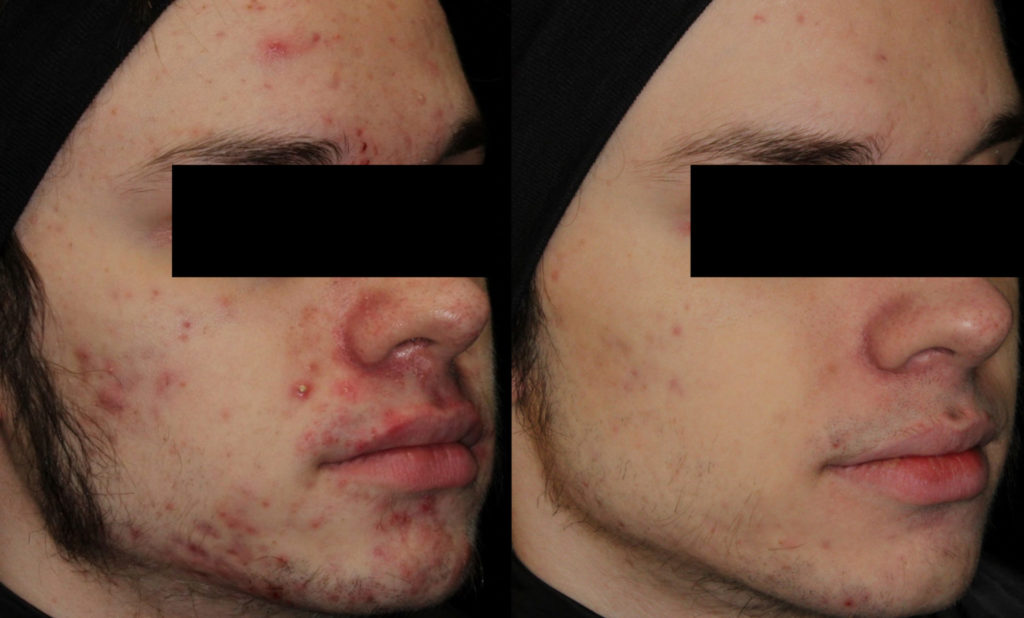
Isotretinoin (best known as Accutane™) is the miracle cure for acne. It is derived from vitamin A and was FDA approved in 1982 for treatment of severe nodular cystic acne. In addition, isotretinoin is also used to treat less severe forms of acne that are unresponsive to traditional therapies, such as those people who fail topical creams/oral antibiotics or who those who initially improve on treatment, but then relapse. Isotretinoin is also indicated for acne that leads to scarring, as well as those people who have become psychologically devastated from their acne. Since it was first approved, millions of Americans have safely taken isotretinoin (Accutane™) with amazing results.
Isotretinoin (Accutane™) is so effective that almost every person who finishes the course of treatment (usually about six months) will experience complete clearance of his or her acne by the end of treatment. Moreover, many people remain clear of acne forever, even after stopping isotretinoin.
The most common side effects of isotretinoin (Accutane™) include dryness of the skin, chapping of the lips, dryness of the eyes, increased risk of sunburn and muscle /joint pains. These side effects are reversible once stopping the medication. Women taking isotretinoin (Accutane™) must not get pregnant during therapy and will require strict monitoring.
Accutane™
There are times when traditional therapies, hormonal therapies, and cosmetic treatments fail to control cystic acne.
Fortunately, there is a miracle cure for acne – Accutane.
How does Isotretinoin (Accutane™) work
Isotretinoin (Accutane™) reverses the underlying pathophysiology of acne through:
- Normalizing sebum (oil) production
- Reducing bacterial counts (C. acnes) on the skin
- Eliminating immune system overactivity (pimples and pus bumps)
- Unclogging of pores-comedolytic
Am I a candidate for Isotretinoin (Accutane™)?
The following people meet criteria for initiation of accutane:
- Moderate to severe inflammatory acne (without scarring) that has not responded to conventional therapy (benzoyl peroxide, topical retinoids, topical and oral antibiotics, and adjunctive treatments).
- Scarring, inflammatory acne inadequately controlled with conventional therapy
- Those who are unable to discontinue oral antibiotic therapy without flaring of their acne.
- Psychological scarring, loss of self-esteem or emotionally bothered from acne.
What are the odds that my acne will be cured from Isotretinoin (Accutane™)?
This depends on several factors. Studies have varied as to the rate of permanent cure, but on average, approximately 55%- 65% of people can achieve permanent cure with one course of accutane. Even if your acne comes back after accutane, the acne often responds to previous therapies that did not work prior to accutane. Some people will need a second course of accutane ultimately.
Factors that increase the risk of relapse of acne after Isotretinoin (Accutane™) include:
- Younger age- those under 16 years of age often require a second course of accutane for complete cure. This does not mean that one should necessarily wait until he /she is older to start accutane, especially if the acne is causing scarring. If the acne is severe enough to warrant accutane, one should still proceed.
- Incomplete course of isotretinoin (those who do not take the full dose)
- Back and chest involvement
- Women with hormonal abnormalities such as PCOS
- Predominance of blackheads rather than pimples, pustules, and cysts.
- Not taking isotretinoin with enough dietary fat (which leads to a significant lack of absorption of the medication).
How do I improve my chances that Isotretinoin (Accutane™) will cure my acne?
- Always take Isotretinoin (Accutane™) with a fatty meal. Since isotretinoin is derived from vitamin A, and since vitamin A is soluble in fat, you must take your medication with enough fat to have proper absorption. The absorption of accutane varies from as little as 20% absorption to as much as 70% absorption depending upon how much fat you ingest when you take your medication. Examples of fat containing foods include a tablespoon of peanut butter, 8 ounces of whole milk, two eggs fried in butter, two strips of bacon, scoop of ice cream, two slices of toast with butter, or a steak. In the original trials, participants ingested 50 g of fat when taking accutane. This would be the equivalent of a Wendy’s baconator!
- Continue taking isotretinoin until your acne completely resolves, and then for an additional 1 to 2 months.
- If possible, take the special preparation of isotretinoin called Absorica LD™. This version has been formulated to be absorbed on an empty stomach. Studies have shown that within two years of taking Absorica LD™, 82% of people did not require any over the counter or prescription medication, and only 5% of people required retreatment with Absorica LD™
Can I take more than one course of Isotretinoin (Accutane™)?
Yes. Some people require a second or third course of accutane to achieve cure. It is advisable to wait at least six months between multiple courses of accutane.
Are there different preparations of isotretinoin?
Yes. The original version is known as Accutane™, produced by Roche pharmaceuticals. Since then, multiple generic versions have been released: Claravis, Amnesteem, Myorisan, Sotret, Absorica, etc.
In 2020, a novel formulation of isotretinoin known as Absorica LD™ was approved. Studies have shown superiority of this version over the others.
Absorica LD has been formulated to be absorbed on an empty stomach. Studies have shown that within two years of taking Absorica LD™, 82% of people did not require any over the counter or prescription medication, and only 5% of people required retreatment with Absorica LD™
Unfortunately, in the state of Michigan, insurance coverage for Absorica LD is often lacking and the out-of-pocket cost would be around $800 a month. Therefore, many patients opt for the generic version.
What are the most common side effects of Isotretinoin (Accutane™)?
The most common side effect of isotretinoin is extreme dryness, particularly of the lips. Most people need to apply lip moisturizers such as Aquafor, Chapstick, and other lip balms multiple times an hour. Your skin will be dry as well and you’ll need to moisturize a couple times a day. Your eyes will become drier and can turn red if you don’t use eyedrops. Artificial teardrops are usually sufficient to treat the dryness. Some people have difficulty wearing contact lenses, though most may continue to wear them. Sometimes, temporary decreased night vision is reported.
At higher doses, you may experience joint aches, muscle aches, and low back pain. For some people, this interferes with physical activity; however, most can tolerate these side effects.
You will also have extreme sensitivity in the sun, and you will be extraordinarily prone to sunburn. Therefore, you will need to take extra precautions if taking isotretinoin (Accutane) in the summer. – Some people defer accutane until the fall and winter because of this side effect.
Your skin will be very sensitive and will heal poorly with surgical procedures for up to 6 months after stopping isotretinoin (Accutane). Therefore, we advise you avoid waxing, tattoos, piercing, elective surgery, and laser procedures while taking Accutane and for 6 months upon completing the course.
Isotretinoin (Accutane™) may cause your acne to flare in the first month or two of treatment, sometimes with severe flaring (pseudo acne fulminans).- This is usually minimized by starting out at a low dose of Accutane for the first month. In addition, pyogenic granuloma skin lesions may rarely develop in acne cysts as well as around fingernails.
Accutane often causes transient elevation of triglycerides and cholesterol and may cause transient elevation of liver enzymes. – For this reason, we recommend minimizing alcohol intake, to no more than two or three drinks a week, while taking accutane. All people require bloodwork monitoring during treatment.
Severe liver toxicity is extraordinarily rare, and Dr. Singer has never had a patient (nor has he ever heard of anyone) who developed irreversible liver damage from Accutane.
Are there serious side effects from Isotretinoin (Accutane)?
1. The most serious side effect is the risk of birth defects to an unborn fetus. For this reason, any female (who can get pregnant) must be on two forms of contraception or be strictly abstinent (for one month prior to the start of Accutane, during the entire course of Accutane, and for one month after completing Accutane). Studies show that fetal exposure to even tiny doses of Accutane results in severe malformations. Women who become pregnant while taking Accutane are strongly advised to terminate the pregnancy. It is important to remember that millions of women who have taken Accutane in their younger years have had no problems with future pregnancies. In general, since the half-life of accutane is about 24 hours, there is no more Accutane in your system once 30 days has elapsed after completing your course.
2. There is controversy as to whether Accutane causes depression and suicide. In 1998, following reports to MedWatch, the makers of Accutane added an additional warning regarding possible adverse effects of “depression, psychosis and rarely, suicide. Following the new labeling however, larger studies failed to demonstrate a convincing link between Accutane and depression. Many studies suggest that severe acne is a risk factor for depression and suicide. Depression is 2 to 3 times more common in patients with acne than those without. And suicidal ideation is also more common in those who have severe acne. In our experience, most people who take Accutane have an improved mood (Accutane may act as an antidepressant for people who are devastated by their acne). A recent meta-analysis of 31 studies concluded that isotretinoin does not appear to be associated with depression and that the treatment of acne reduces symptoms of depression. Over the past 20 years, Dr. Singer has had a few patients who became moody and tearful or became angered easily after starting Accutane. These symptoms resolved immediately after discontinuing Accutane. If someone has a history of depression, and it is well-controlled/stable, Dr. Singer will prescribe Accutane if he works closely with the patient’s mental-health provider.
3. Accutane has also been implicated as a ‘potential’ cause of inflammatory bowel disease, in the form of either Ulcerative colitis or Crohn’s disease. The data regarding Accutane use as a cause of inflammatory bowel disease is conflicting. Some studies have shown no association while others have shown 2.5x increased risk. No definitive cause and effect has ever been proven. Even in reports that demonstrate a possible causal association, the overall risk of inflammatory bowel disease in patients who take accutane is very small.
The current American Academy of Dermatology position published in 2010:
‘Current evidence is insufficient to prove either an association or a causal relationship between isotretinoin use and inflammatory bowel disease in the general population. While some recent studies have suggested such a relationship, further studies are required to conclusively determine if an association or causal relationship exists and/or whether inflammatory bowel disease risk may be linked to the presence of severe acne itself.’
In summary, there is no current definitive evidence to support a link or risk of inflammatory bowel disease from Accutane.
4. Accutane may rarely cause a condition called benign intracranial hypertension in which increased amounts of fluid develop around the brain leading to headaches and nausea. Fortunately, this is a very rare side effect. Dr. Singer has only seen this once during his career.
Can I be in the sun while taking Isotretinoin (Accutane™)?
We tell people that you are five times more likely to burn in the sun while taking accutane.
You may be in the sun, if you practice very good sun protection including protective clothing, hats, frequent application of broad-spectrum sunscreen 50+ SPF, with reapplication every two hours. Seeking shade is optimal.
Many people choose to defer the course of accutane until the fall and winter.
Can I drink alcohol while taking Isotretinoin (Accutane™)?
Only to a limited amount. We recommend only two or three alcoholic beverages per week. Drinking too much alcohol while taking accutane will produce irritation of the liver.
How long is the average course of Isotretinoin (Accutane™)?
Six months. Some require an extra month or two. This will depend on how you tolerate the side effects and how quickly we can proceed with the dosing. In general, we recommend treating until the acne is completely clear and then for one to two additional months to prevent relapse.
Will I require bloodwork monitoring during Isotretinoin (Accutane™)?
We do require baseline fasting blood work before starting Accutane. And we will require additional bloodwork every month/ every other month during Accutane. – this depends on dosing and whether patient has had any bloodwork abnormalities.
Women of childbearing potential will also need monthly pregnancy testing. This may be performed through either urine or blood. Home pregnancy testing is now permitted during the COVID-19 outbreak.
Will Isotretinoin (Accutane™) make my acne worse before it gets better?
Oftentimes, acne can flare at the start of isotretinoin. This is referred to as ‘pseudo-acne fulminans.’ This can occur since the current treatments (i.e. oral antibiotics) partially controlling the acne are discontinued. Also, the isotretinoin itself may initially flare the acne before it improves it.
For this reason, we recommend starting Isotretinoin (Accutane™) at a lower dose for the first month, and gradually increasing therapy to minimize any significant flaring. On occasion oral prednisone and oral Bactrim are required at the initiation of isotretinoin to minimize flaring.
True acne fulminans is a very rare complication requiring aggressive management.
What forms of birth control are permissible?
Two forms of contraception (one primary form and one secondary form) are required unless the female agrees to be strictly abstinent. Please see the I pledge program for further information.
What is the I pledge program?
The iPLEDGE Program is a computer-based risk management program designed to further the public health goal to eliminate fetal exposure to isotretinoin through a special restricted distribution program approved by the FDA. The program strives to ensure that:
- No female patient starts isotretinoin therapy if pregnant
- No female patient on isotretinoin therapy becomes pregnant
This enhanced program is a SINGLE pregnancy risk management program for prescribing and dispensing all isotretinoin products (brand and generic products). The iPLEDGE Program requires registration of all wholesalers distributing isotretinoin, all healthcare professionals prescribing isotretinoin, all pharmacies dispensing isotretinoin, and all male and female patients prescribed isotretinoin. This program is designed to create a verifiable link between the negative pregnancy test and the dispensing of the isotretinoin prescription to the female patient of reproductive potential. The iPLEDGE Program requires that all patients meet qualification criteria and monthly program requirements. Before the patient receives his/her isotretinoin prescription each month, the prescriber must counsel the patient and document in the iPLEDGE Program system that the patient has been counseled about the risks of isotretinoin.
There are also additional qualification criteria and monthly requirements for female patients of reproductive potential.
As part of the ongoing risk management of isotretinoin products, it is crucial that a female of reproductive potential selects and commits to use two methods of effective contraception simultaneously for one month before, during, and for one month after isotretinoin therapy. She must have 2 negative urine or blood (serum) pregnancy tests with a sensitivity of at least 25 mIU/ml before receiving the initial isotretinoin prescription. The first pregnancy test is a screening test and can be conducted in the prescriber’s office. The second pregnancy test must be done in a CLIA-certified laboratory according to the package insert. Each month of therapy, the patient must have a negative result from a urine or blood (serum) pregnancy test conducted by a CLIA-certified laboratory prior to receiving each prescription. During the COVID pandemic, the I pledge program has relaxed restrictions, enabling home pregnancy testing if needed.